

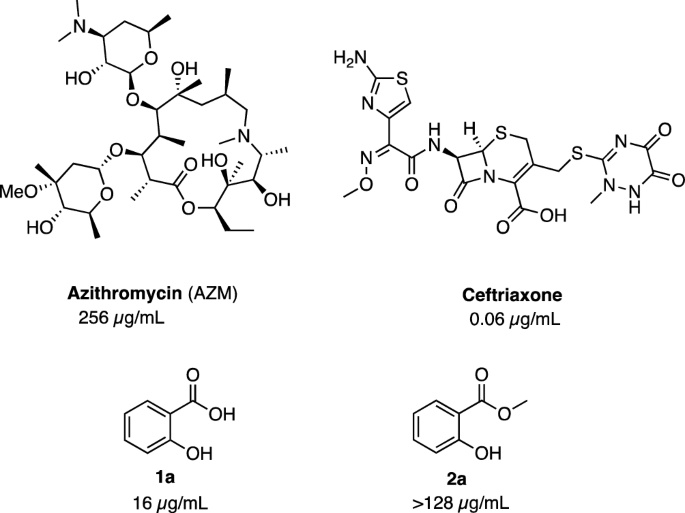
2014), and eravacycline, a fully synthetic fluorocycline discovered at Tetraphase Pharmaceuticals (Watertown, MA) ( Clark et al. In recent years, two new tetracyclines have entered clinical development: omadacycline, a semisynthetic aminomethylcycline derivative of minocycline discovered at Paratek Pharmaceuticals (Boston, MA) ( Draper et al. Tigecycline continues to be an important treatment option for serious infections caused by pathogens resistant to other antibiotic classes. Tigecycline, a semisynthetic parenteral glycylcycline, was discovered in 1993 by scientists at Lederle (which later became Wyeth, New York), and introduced into clinical use in 2005 ( Sum and Petersen 1999 Zhanel et al. The core structure rings ( A– D) and carbons (1–12) are labeled in the chemical structure of tetracycline using the convention for tetracycline carbon numbering and ring letter assignments.Īfter a long pause in the advancement of the tetracycline class, renewed interest in optimization of tetracyclines during the late 1980s led to the discovery of semisynthetic derivatives with improved potency against difficult-to-treat emerging multidrug-resistant (MDR) Gram-negative and -positive pathogens, including bacteria with tetracycline-specific resistance mechanisms. Tetracycline structures are labeled with generic names trade names and year of discovery are indicated within parentheses. 1).Ĭhemical structures of clinically used tetracyclines and development candidates. Several of these “legacy” tetracyclines remain in clinical use for the treatment of uncomplicated respiratory, urogenital, gastrointestinal, and other rare and serious infections however, the dissemination of tetracycline-resistant mechanisms has narrowed their utility, limiting use to only infections with confirmed susceptibility ( Fig. Other tetracyclines that followed over the next two decades were also natural products produced by streptomycetes (tetracycline, demethylchlortetracycline) or semisynthetic derivatives with improved antibacterial potency, spectrum, resistance coverage, solubility, and/or oral bioavailability (methacycline, rolitetracycline, lymecycline, doxycycline, and minocycline) ( Jarolmen et al. Food and Drug Administration (FDA) in 1950 and marketed as Terramycin ( Finlay et al. Soon after, Pfizer (New York) scientists isolated oxytetracycline, approved by the U.S. Chlortetracycline, produced by Streptomyces aureofaciens, and marketed as Aureomycin, was first reported by Benjamin Duggar at Lederle Laboratories in 1948 and approved for clinical use that same year ( Duggar 1948). The first tetracyclines were natural products derived from the fermentations of actinomycetes.
Tetracycline antibiotics are well known for their broad spectrum of activity, spanning a wide range of Gram-positive and -negative bacteria, spirochetes, obligate intracellular bacteria, as well as protozoan parasites.
#E coli gram positive or negative full
New chemistry approaches have enabled the creation of synthetic derivatives with improved in vitro potency and in vivo efficacy, ensuring that the full potential of the class can be explored for use against current and emerging multidrug-resistant (MDR) pathogens, including carbapenem-resistant Enterobacteriaceae, MDR Acinetobacter species, and Pseudomonas aeruginosa. Since the discovery of the first tetracyclines more than 60 years ago, ongoing optimization of the core scaffold has produced tetracyclines in clinical use and development that are capable of thwarting many of these resistance mechanisms. As with all antibiotic classes, the antimicrobial activities of tetracyclines are subject to both class-specific and intrinsic antibiotic-resistance mechanisms.
Tetracyclines possess many properties considered ideal for antibiotic drugs, including activity against Gram-positive and -negative pathogens, proven clinical safety, acceptable tolerability, and the availability of intravenous (IV) and oral formulations for most members of the class.


 0 kommentar(er)
0 kommentar(er)
